Overview
Our work explores the fascinating and complex system of nitrogenase, a unique metalloenzyme responsible for converting atmospheric nitrogen (N₂) into ammonia (NH₃), a crucial step in the global nitrogen cycle. By combining molecular biology, biochemistry, and structural insights, we aim to unravel the assembly, function, and evolutionary history of nitrogenase, while pushing the boundaries of heterologous expression and synthetic biology.
1. Heterologous Expression of Nitrogenase
Nitrogenase consists of two components:
- The Fe protein (NifH), a reductase that delivers electrons.
- The MoFe protein (NifDK), a catalytic complex containing two intricate metalloclusters: the [Fe₈S₇] P-cluster and the [(R-homocitrate)MoFe₇S₉C] M-cluster (FeMo-cofactor).
Reconstituting an active nitrogenase outside its native diazotrophic host (Azotobacter vinelandii) has long been a challenge due to the complexity of the metallocluster biosynthesis. We addressed this by implementing a stepwise “divide-and-conquer” approach, heterologously expressing and assembling nitrogenase components in Escherichia coli.
Our research demonstrated:
- Successful expression of a fully active NifH protein, which assembled its essential [Fe₄S₄] cluster in E. coli with co-expression of maturation factors.
- Biosynthesis of P- and M-cluster-containing proteins, validating cluster assembly pathways via biochemical and spectroscopic analyses.
- Successful diazotrophic growth in E. coli, demonstrating the functional activity of heterologously expressed nitrogenase.
This work provides a prototype system for nitrogenase expression, offering pathways for future biotechnological applications like sustainable ammonia production.
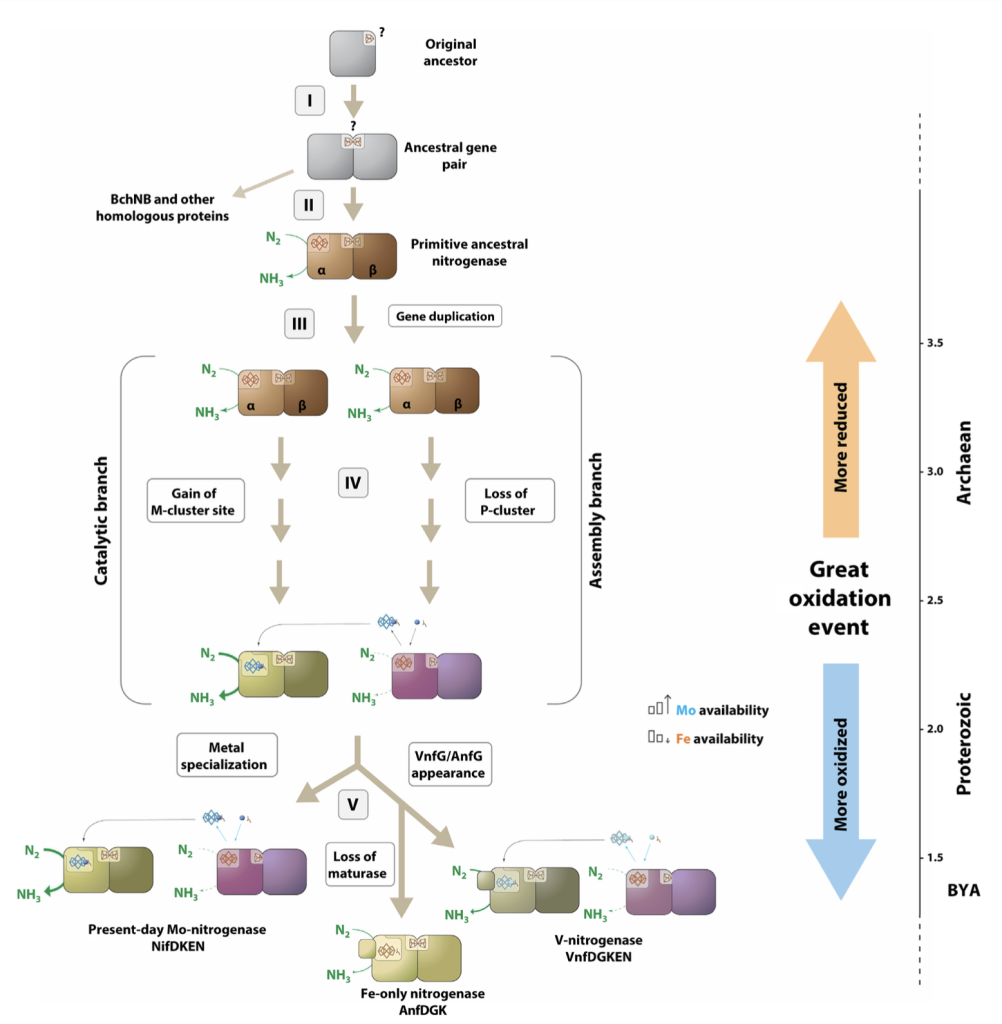
2. Nitrogenase Evolution: A Look into the Past
Beyond function, nitrogenase holds clues to its own origins. In collaboration with colleagues, we investigated NifEN, the maturase that synthesizes the M-cluster before delivering it to NifDK. Remarkably, NifEN itself showed residual nitrogen-fixing activity, suggesting it might represent an ancient prototype nitrogenase.
This finding resolves a long-standing paradox: How did the first nitrogenase cofactor evolve without a maturase? NifEN-like ancestors, with their simpler [Fe₈S₉C] L-cluster, likely acted as early nitrogenases, predating the Mo-containing cofactor we observe today. Our work sheds light on how evolution co-opted a primitive enzyme into a fully functional nitrogenase.
Building on our earlier findings, we recently expressed NifEN in E. coli together with only NifUSB and NifMH, but without NifDK or the full suite of nitrogenase biosynthetic genes. Remarkably, the system retained catalytic activity under ambient conditions. This demonstrates that NifEN can function as a minimal nitrogenase-like enzyme when paired with just NifH, reinforcing its proposed role as an ancestral prototype. It also brings us closer to building a streamlined, sustainable diazotrophic pathway with fewer components and a smaller genetic footprint.
Why It Matters
Nitrogenase research bridges fundamental science and real-world applications. From understanding life’s evolutionary history to enabling synthetic biology for agriculture and energy, this work opens avenues for innovation. By mimicking nature’s solutions, we can strive for sustainable alternatives to industrial nitrogen fixation.
Publications:
- Y. A Liu, C. C. Lee, K. Górecki, M. T Stiebritz, C. Duffin, J. B. Solomon, M. W. Ribbe, Y. Hu, Heterologous synthesis of a simplified nitrogenase analog in Escherichia coli, (2025), Science Advances, 11(18). Read
- C. C. Lee*, K. Górecki*, M. Stang, M. W. Ribbe, and Y. Hu, Cofactor maturase NifEN: A prototype ancient nitrogenase?, (2024), Science Advances, 10(24). Read
- J. B. Solomon*, Y. A. Liu*, K. Górecki*, R. Quechol, C. C. Lee, A. J. Jasniewski, Y. Hu, Markus W. Ribbe, Heterologous expression of a fully active Azotobacter vinelandii nitrogenase Fe protein in Escherichia coli, (2023), mBio, 14:e02572-23. Read
- R. Quechol, J. B. Solomon, Y. A. Liu, C. C. Lee, A. J. Jasniewski, K. Górecki, P. Oyala, B. Hedman, K. O. Hodgson, M. W. Ribbe, and Y. Hu, Heterologous synthesis of the complex homometallic cores of nitrogenase P- and M-clusters in Escherichia coli, (2023), Proceedings of the National Academy of Sciences USA, 120 (44) e2314788120. Read (open access at PubMed)
- M.W. Ribbe, K. Górecki, M. Grosch, J.B. Solomon, R. Quechol, Y.A. Liu, C.C. Lee, and Y. Hu, Nitrogenase Fe Protein: A Multi-Tasking Player in Substrate Reduction and Metallocluster Assembly, (2022), Molecules, 27, 6743. Read